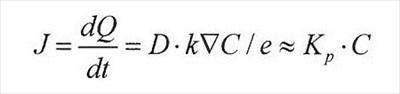Voluntary Guideline on Impurities in Monograph OTC Topicals Excluding NDA and ANDA Products
Introduction
1.1 Objective of the Guideline
This white paper provides guidance for the evaluation and reporting of impurities in OTC monograph drug products formulated as topicals and topical rinses. Topicals and topical rinses are not administered per discrete doses and they exhibit complex tissue interactions precluding the development of a single model to regulate impurities. This document serves as the Consumer Healthcare Products Association’s (CHPA) member company consensus on this complex issue.
1.2 Background
CHPA formed a sub-committee to address issues regarding the handling of impurities in OTC topical products covered by the CFR monograph system (21 CFR Part 330). Currently, available guidance is based on new drug entities that differ from monograph OTC topical products in that monograph OTC products have a long market experience, may have active pharmaceutical ingredients (APIs) without specific structure-function relationships, and have specific effects based on route of administration. Due to the large body of data that supports monograph OTC products, appropriate guidelines should be developed that take into account the major distinctions between OTC and new drug entities as well as the complications associated with dermal administration.
Monograph OTC topical drug products are widely distributed, generally recognized as safe/effective (GRAS/GRAE), have years of market experience and have a well-characterized, historical record of adverse events. "GRAS/GRAE" is a designation for drugs that are generally recognized, among qualified experts, as having been adequately shown to be safe/effective under the conditions of their intended use. 21 CFR 170.3(i) defines "safe" as a material with a “reasonable certainty in the minds of competent scientists that the substance is not harmful under its intended conditions of use”. Also, most topical OTC consumer products are based on core formulations that have been well characterized by the manufacturer with minor excipient variations for consumer appeal (e.g. fragrance, flavor, color). Finally, market experience accrued over the long life of the products, along with quantitative structure-activity relationship (QSAR) characteristics, estimated bioavailability, and consumption by the population, has provided foundational evidence of the safety of monograph OTC topical drug products.
Monograph OTC topical products may contain APIs that are single molecular entities or “atypical actives” with varied structures and no specific structure-function relationship (such as aloe-vera, petrolatum, or witch hazel). The fact that such compounds do not have discrete active compounds makes impurity qualification challenging. Additionally, topical OTC products are frequently non-dosage-limited with multiple APIs and can be applied to various tissue types. Current guidances make no allowance for the route of administration when considering limits on impurities.
The complexities due to the above factors prevent a single general regulation governing impurities arising from drugs formulated in monograph OTC topical products. Therefore, control strategies for impurities should be developed using a coherent, scientifically based approach on a per formula basis.
1.3 Scope of the Guideline
This document serves as a consensus on the appropriate approach to the handling of impurities in FDA monograph topical OTC drug products. Topical OTC drug products cover a breadth of formulations, each with different active ingredient amounts, drug purposes, indications, and other legal requirements for marketing under monograph status. Ref CFR 201.66
Given the complexity of monograph topical OTC formulations, this white paper will only address impurities arising from APIs in topicals and topical rinses. APIs are the chemically active moieties, the drug substances used in GRAS/GRAE drug products in the United States, and are marketed under one of the following regulatory classifications (CHPA Your Health At Hand Book, October 2010):
- Category I ingredients - (GRAS/GRAE for the claimed therapeutic indication) contained in a tentative final monograph or a final monograph (FM).
- Category III ingredients - (insufficient data available to permit final classification) contained in marketed products such as ingredients are referred to as “FM pending.”
Monograph drug product impurities addressed in this document include degradation products of the drug substance, reaction products of the drug substance with other drug substances in the formulation, reaction products of the drug substance with an excipient and/or the immediate container closure system (collectively referred to as “degradation products”). All impurities will be considered except the following: impurities arising solely from excipients present in the new drug product and/or extracted or leached from the container closure system, impurities revealed in the course of clinical development, and impurities arising from residual solvents and heavy metals as these are well defined in the USP. Related compounds of the ingredients that are not degradants are controlled in the raw materials and not in the finished product.
Approach to Thresholds
Each manufacturer is responsible for developing limits for the degradation products observed during manufacture and/or stability studies of their OTC monograph products. These limits should be based upon sound scientific appraisal of potential and observed degradation pathways. In addition, the manufacturer should summarize laboratory studies conducted to detect degradation products and any analytical procedures developed for those degradation products that literature references indicate to be unusually potent based on structure, producing toxic or significant pharmacological effects.
Manufacturers should establish limits for degradants in monograph OTC topical products with support based on consideration of the following factors:
- Compendial limits for API raw material
- Limits for impurities in the product should scale with compendial impurities limits for the API, where compendial limits exist
- Applicability of limits for topical administration
- Consumer exposure to the product
- Annual number of doses of product
- Special sensitivity of the target population (e.g. pediatric, elderly, diabetics)
- Market experience
- Support with epidemiological data if available
- Trends of data
- Adverse events(AEs) associated with product
- Data relating AEs to actives, excipients, or degradants
- Length of market history
- Similarities of limits to those in NDA or ANDA products
- Similarities of limits to those in products approved by other health authorities
- Support with epidemiological data if available
- Duration and frequency of the exposure
- Ease of product absorption through the skin
- Directions for use mitigate exposure to impurities in product
- Estimation of the systemic exposure per day (i.e. “rinse-off” products provide lower exposure than “leave-on” products.
- Route of administration and bioavailability
- Ease of product absorption through the skin
- Metabolism of product constituents in the dermis
- Estimation of the systemic exposure per day
- QSARs
- Toxicity data available for compounds with similar structures
- Dermal toxicity and toxicokinetic data
- Photo-irritation/photosensitization data
- Toxicity data available for compounds with similar structures
- Core formulations and similarities to other products
- History of use of similar formulations
- Gap analysis of formulas
As manufacturers work on a case-by-case basis to develop impurity limits for monograph OTC topical products, considerations must also be made for the specifics described in Appendix I.
In conclusion, this white paper provides guidance for the evaluation and reporting of impurities in OTC monograph drug substances formulated as topicals and topical rinses. A single model to regulate impurities cannot be developed for these products because they are not administered in discrete doses, may have multiple APIs, and exhibit complex tissue interactions. Consequently, this document serves as a CHPA member company consensus regarding the handling of this complex issue.
Appendix I
Topical and topical rinse OTC monograph drug products are complex formulations with numerous OTC variants that prevent the development of a single, robust model that accounts for all associated impurities. The complexity arises from critical physicochemical factors that impact the drug’s absorption and bioavailability. Therefore, a rational approach that takes these factors into consideration will provide a clear scientific foundation for addressing impurity limits specific to a given OTC topical or topical rinse product:
Absorption variability – Absorption rate estimation for topical and topical rinses are governed by a simple model — Fick’s first law of diffusion at steady state:
where dQ/dt is the rate of chemical absorbed, D represents the diffusivity in the stratum corneum), k is the stratum corneum/vehicle partition coefficient, is the concentration gradient above and below stratum corneum, e (the thickness of the stratum corneum), Kp is the permeability coefficient, and C is the applied chemical concentration.
Fick’s law illustrates that both the size and charge of given chemical entity has a pronounced effect on its absorption rate. While calculating absorption is not essential for every OTC topical product, Fick's Law helps to estimate the possible exposure to an impurity. For example, a lipophilic compound will absorb more readily through the skin than a hydrophilic one because the transfer process used is passive diffusion. Also, smaller molecular weight compounds may have a different dermal retention time than larger compounds (i.e. smaller compounds will move through the skin more quickly than larger ones). Nonpolar compounds will be absorbed at a rate directly proportional to their lipophilicity and inversely proportional to their size. Also, hydrophilic compounds will use appendages such as follicles to transfer across the dermal layer so follicular density should be factored to appropriately assess topical absorption.
Tissue variation (i.e. thickness, type, vascularity) – A primary factor limiting absorption through the skin is the stratum corneum. This outermost skin layer is composed of keratinocytes with thickened cell walls and a dry, keratinous intracellular matrix which prevents fluid loss through the skin and prevents absorption of many xenobiotics. Additionally, if a compound penetrates the stratum corneum, it then must traverse through six distinct layers of skin before entering systemic circulation and becoming bioavailable. The thickness of the stratum corneum varies from one region of the body to another resulting in different absorption rates. Stratum corneum on the palms of the hands and soles of the feet is very thick making absorption across these areas difficult. However the skin on the scrotum has a thin stratum corneum making it fairly easy for impurities to penetrate the skin. The hydration state, temperature, and integrity of the stratum corneum can also impact penetration of the skin. When the stratum corneum is hydrated (normally 7% water by weight), absorption occurs to an approximately 10-fold greater extent than when completely dry. Also, when in contact with water, penetration across the stratum corneum can approximately triple. An increase in temperature will increase dermal blood flow and increase dermal absorption. Damaged or compromised skin (i.e. burns, caustic agents, cuts/abrasions) that no longer has a completely intact stratum corneum has increased permeability.
Another consideration would be topical products that are applied close to the oral cavity (e.g. lips, perioral). These have a greater potential for systemic absorption due to the highly vascular oral mucosa that provides a direct route into systemic circulation and whole body distribution.
Solvents present in a formulation can also impact dermal absorption. Typically less vehicle-soluble compounds will penetrate the skin more easily than compounds that are more soluble in the vehicle. Also, chemicals that increase surface permeability (e.g. dimethyl sulfoxide) results in increasing dermal absorption.
Physical removal of drug substance from application site – Topicals can be inadvertently removed by washing the treated area or rubbed off by clothing, but they can also be removed purposefully as directed for some products. This results in a highly variable total exposure to the topical and its impurities due to uncontrollable and controllable factors.
Exposure duration variability (e.g. rinse off vs. leave on) – Different topical products have different directions for use. Some will remain on the skin for days (leave on) while others may only be in contact with the skin for several minutes. Such instructions result in highly variable exposure to products and their impurities. Impurities in leave-on products will have greater exposure.
Atypical drug components and natural compounds – Atypical compounds such as petrolatum have varied structures and no specific structure-function relationships. These types of compounds do not have discrete related compounds thus challenging a robust determination of their impurity profile.